The authorisation of medicinal products within the EU requires an environmental risk assessment (ERA) for the active substance(s) contained therein. The aim of the environmental risk assessment is to evaluate and limit potentially adverse effects on the environment and public health. As Module 1.6.1 (for non-GMOs), an ERA is part of the authorisation dossier.
The current guideline (EMEA/CHMP/SWP/4447/00 Rev. 1-Corr.) has been in force since 1 September 2024. It represents a significant expansion of the previously valid guideline, for example by taking into account the risks of antimicrobial resistance, critical active substances (such as endocrine-active substances and antiparasitics) and accumulation via the food chain. It can be assumed that the revision of the Medicines Directive will result in a further tightening of the requirements for environmental risk assessment.
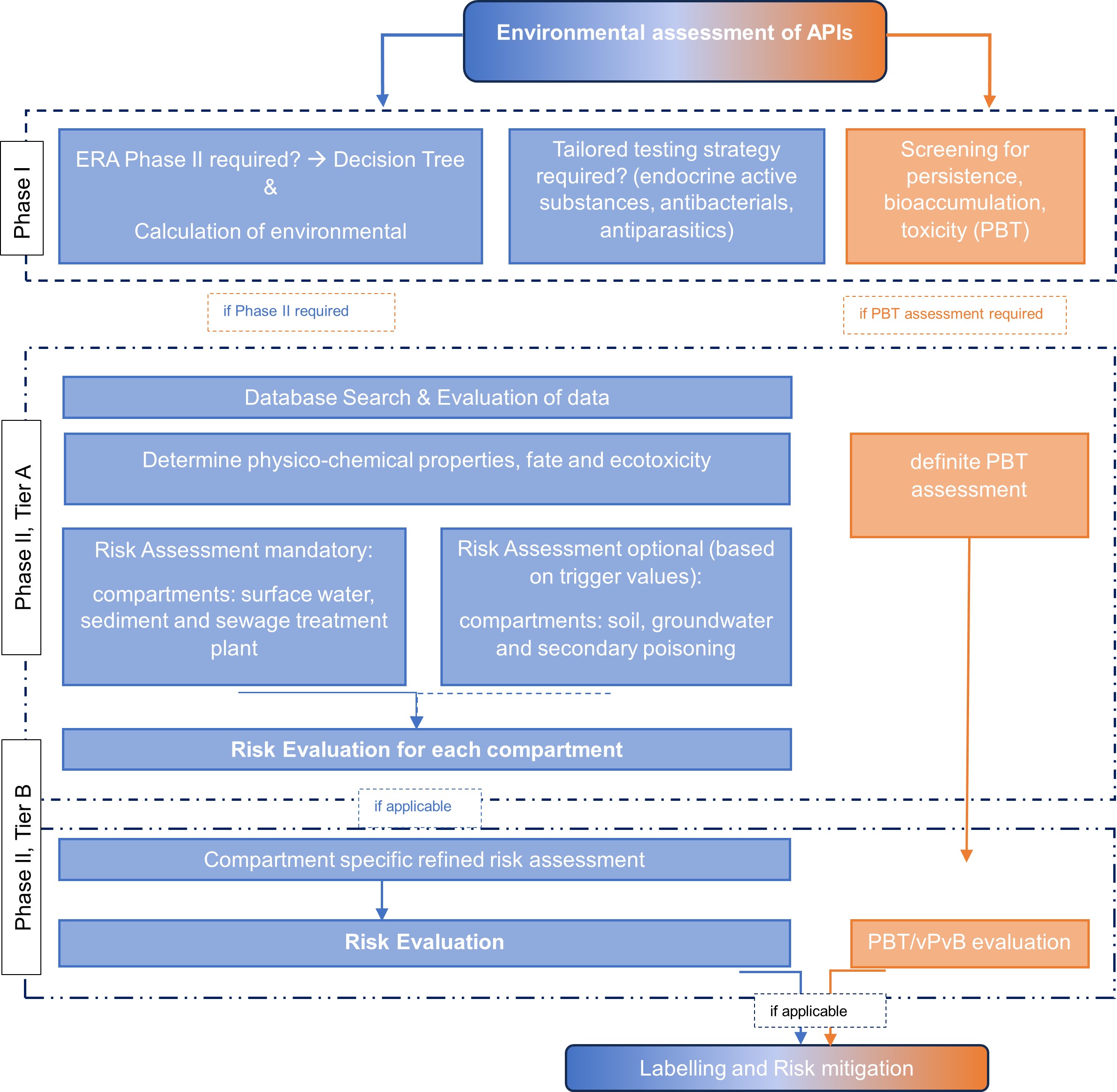
The procedure for an ERA based on a flow chart is shown below:
Our services
We support you in preparing a comprehensive ERA Module 1.6.1 for human medicinal products based on literature data and experimental studies:
- Assessment of the necessity and scope of an ERA
- Request for originator data
- Comprehensive database search in accordance with the requirements of the new guideline
- Evaluation of existing literature data for relevance (CRED method)
- Data gap analysis and development of a test strategy, in consultation with the authority if necessary
- Study initiation and study monitoring in line with ERA requirements
- Preparation of M1.6.1
- Communication with the authority
Thanks to our close cooperation with various certified testing laboratories, we can offer you the complete package from a single source (ERA Service List).
We also provide training twice a year in our practical online seminar!
Contact us for further information:
Bianca Leubner
Phone: +49 (0) 341 223 292 36
email
Environmental risk assessment of medicinal products for human use

